All published articles of this journal are available on ScienceDirect.
Utilising Self-acupressure to Manage Type 2 Diabetes Mellitus Control Parameters
Abstract
Background
In Indonesia, only two-thirds of patients diagnosed with diabetes mellitus (DM) are projected to receive both non-pharmacological and pharmaceutical treatment. The majority of patients receiving the medication do not achieve adequate control of DM.
Objective
The purpose of this study was to examine the impact of self-administered acupressure on type 2 DM (T2DM) management parameters, specifically targeting ST36, LR3, KI3, and SP6 acupuncture points.
Methodology
This study utilised an experimental research design and included a total of 25 patients with diabetes mellitus from the Gianyar Public Health Centre. Participants were selected using a simple random sampling technique. The individuals underwent a 13-week training program to learn self-acupressure techniques targeting certain areas of their bodies, including Zusanli (ST36), Taichong (LR3), Taixi (KI3), and Sanyinjiao (SP6). The control parameters of diabetes mellitus were examined both before and after therapy.
Results
The analysis revealed significant differences in the p-values of various control parameters before and after self-acupressure. Specifically, the p-values for HbA1c, LDL, BMI, systolic pressure, fasting blood glucose, and 2 hours post-prandial glucose were found to be p<0.001, indicating self-acupressure, as a complementary therapy, has an impact on these parameters. However, the p-value for diastolic pressure was 0.57, suggesting that self-acupressure did not have a significant effect on this parameter.
Conclusion
The application of self-acupressure at certain points such as ST36, LR3, Taixi, and SP6 has been proven to effectively reduce the control parameters of diabetes mellitus including HbA1c, LDL, BMI, systolic pressure, and fasting blood glucose levels. It is recommended that those who are enrolled in the complementary traditional health care program in the community provide advice on how to perform self-acupressure in diabetic patients.
1. INTRODUCTION
Diabetes mellitus (DM) is a persistent metabolic condition resulting from insufficient insulin production by the pancreas or ineffective utilisation of the insulin produced by the body [1]. Both genetic factors and lifestyle choices impact these diseases. According to PERKENI, there is a correlation between lifestyle changes and the increase in the number of instances of type 2 DM (T2DM) [2]. According to the IDF Diabetes Atlas in 2021, around 10.5% of adults aged 20-79 have diabetes, and nearly half of them are ignorant of their illness. IDF forecasts that by 2045, about 783 million adults, or 1 in 8, will have diabetes, representing a 46% rise [3].
Basic health research in 2018 in Indonesia indicated a substantial increase in the prevalence of DM in the country [4]. In 2013, the incidence of DM in Indonesia was 6.9%, which subsequently increased to 8.5% in 2018. The projected number of individuals with DM in Indonesia exceeds 16 million, regarding the country's entire population. Indonesia's ranking as the sixth country with the highest number of T2DM sufferers in the world is based on this statistic, following China, India, the United States, Brazil, and Mexico [4]. According to the Bali Provincial Health Office, in 2018, T2DM cases in Bali were ranked ninth out of the ten most common cases that require outpatient treatment [5]. It is concerned that T2DM cases ranked first among the top 10 diseases in outpatients from all public hospitals in the Gianyar Regency in 2016, according to Gisnyar District Health Reports in 2018 [6].
DM poses a significant risk to health due to the numerous complications it can lead to. The World Health Organisation (WHO) has reported that DM has emerged as a significant contributor to vision loss, kidney dysfunction, cardiac events, cerebrovascular accidents, and lower limb amputations. According to the WHO, 2 million deaths worldwide in 2019 were attributed to DM [1].
In addition to the risk of complications and high death rates, T2DM also imposes a substantial economic burden. T2DM's financial expenses have experienced a significant growth of 316% in the past 15 years. According to the International Diabetes Federation, managing T2DM costs 966 billion USD [7]. According to the Indonesian National Health Insurance (JKN), the money allocated to Diabetes services in Indonesia was IDR 700.29 billion in 2014. This sum increased to IDR 1.877 trillion in 2017, as reported by the Ministry of Health [8].
The Indonesian Endocrinology Association (Perkeni) clarified that the desired level of glycaemic control has not been achieved successfully [2]. Most people with T2DM who take the medication are not effectively managed. Perkeni establishes various metrics to control diabetes mellitus. T2DM is considered well controlled when specific target parameters are met. These parameters include a body mass index (BMI) between 18.5 and 22.9 kg/m2, pre-prandial capillary blood glucose levels between 80 and 130 mg/dl, postprandial (PP) capillary blood glucose levels below 180 mg/dl after 2 hours, haemoglobin-glycosyl (HbA1c) levels below 7%, blood pressure below 140/90 mmHg, low-density lipoprotein (LDL) below 100 mg/dl, triglyceride levels below 150 mg/dl, and high-density lipoprotein (HDL) cholesterol levels above 40 mg/dl for males and above 50 mg/dl for females [2].
Regarding the parameters established by Perkeni, certain outcomes demonstrated unacceptable results. A study conducted at the Bahu Health Centre in Manado City examined customers with T2DM. The findings revealed that a significant majority (77.3%) of these clients had HbA1c values (>7%) that were not under control [9]. According to the BMI analysis, it was determined that 68.6% of people with diabetes did not achieve the desired BMI range of 18.5-23. According to Pardede et al., 39.3% of T2DM clients did not achieve the goal blood pressure [10].
The inadequate achievement of the T2DM control objectives in Indonesia is mainly attributed to the chronic nature and progressive course of T2DM. Current therapies have not achieved complete restoration of the pancreas' function as an organ that produces insulin. Complementary medicine can be used in the public to manage T2DM [11]. This includes the use of herbal medicines, medicinal plants, yoga therapy, mindfulness therapy, and acupressure [12]. Complementary medicine (e.g. natural herbal, yoga, mind-body practices) effectively regulates blood glucose levels in individuals with diabetes mellitus [13]. Acupressure induces the release of neurotransmitters by stimulating the neurones, hence activating the hypothalamus to regulate the activity of the endocrine gland [14].
A previous study found acupressure therapy at the Zusanli point (ST36) for 30 minutes every visit over a period of 11 weeks to be beneficial in lowering blood glucose levels of T2DM patients [14]. A Randomized Clinical Trial study on Chinese applied acupressure to the Zusanli points (ST36), Taichong (LR 3), Taixi (KI 3), and Sanyinjiao (SP 6) bilaterally in T2DM patients for five minutes each demonstrated a significant increase in insulin levels and decrease blood glucose level [15]. The study on self-acupoint massage (SEAM) on T2DM patients discovered that after 12 weeks of receiving SEAM, the observation group experienced a significant decrease in glycosylated haemoglobin (HbA1c) levels, from 8.35% ± 1.84% to 7.29% ± 1.38% (p < 0.01) [16].
Given that T2DM is a chronic and incurable condition that typically worsens over time, it is important to promote the client's autonomy in managing the disease. Further studies and research are required to investigate the creation of a procedural model for self-administered acupressure as a supplemental therapy for managing T2DM.
1.1. Objectives
The objective of this study was to investigate the impact of acupressure on the control parameters of T2DM, namely, HbA1c, LDL, BMI, systolic pressure, diastolic pressure, fasting blood glucose, and 2 hours postprandial blood glucose levels. Acupressure was applied inde- pendently at the Zusanli (ST36), Taichong (LR 3), Taixi (KI 3), and Sanyinjiao (SP 6) acupuncture points.
2. METHODS
2.1. Study Design
This study was carried out in the Ubud Public Health Centre in Gianyar Regency, Bali Province, Indonesia, from July to October 2023. This research followed a one-group pretest-posttest design.
2.2. Sample
This study included 65 individuals with T2DM from the Ubud Public Health Centre. Participants were chosen using a simple random sample technique. The study included individuals who lived near Ubud Health Centre, had diabetes mellitus (DM), faced challenges with one or more DM control measures (HbA1c, LDL, BMI, systolic pressure, diastolic pressure, fasting blood glucose, and 2 hours postprandial blood glucose levels), and possessed the ability to read and write. However, individuals with additional health conditions such as kidney disorders, cardiovascular diseases, nephropathy, and stroke, as well as those with psychiatric disorders, were excluded from the study (Fig. 1).
2.3. Data Collection and Intervention
The principal investigator collected the list of patients with type 2 diabetes who actively participated in PROLANIS (chronic disease management programme in Indonesia) from three public health centres. Then, we randomly selected them to give them a similar chance. Online software was used for randomisation. A total of 31 respondents were selected for the intervention. During the intervention and post-test, six respondents were lost to follow up. The final analysis was for 25 respondents.
The initial data collection promptly ensues. Before the intervention, the control parameters of DM were initially measured. Blood glucose levels were tested using a glucometer, both during fasting and two hours after eating. The clinical laboratory analysed the HbA1c and LDL values. Digital metres and scales were used as tools to assess the nutritional status. The digital sphygmomano- meter was used to measure both systolic and diastolic blood pressure.
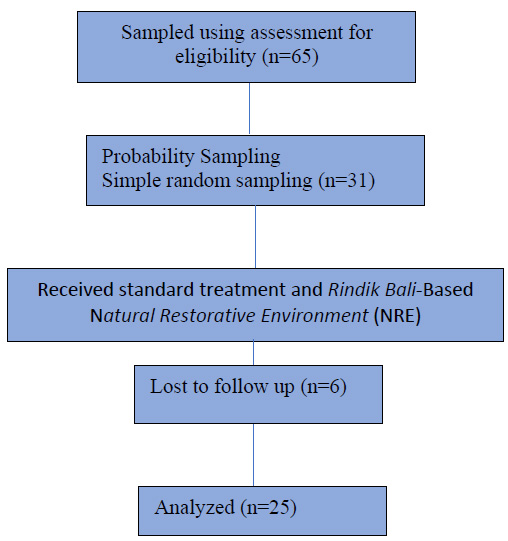
Sample flow.
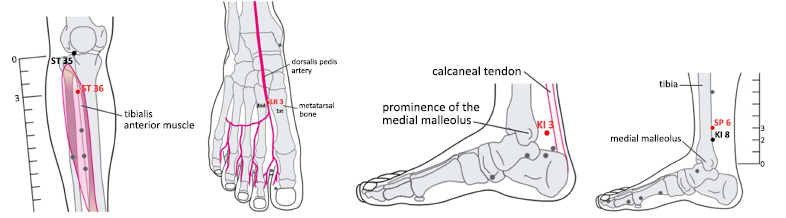
Furthermore, participants were instructed to perform self-acupressure exercises at specific points of acupunc- ture, including Zusanli (ST36), Taichong (LR 3), Taixi (KI 3), and Sanyinjiao (SP 6). The precise anatomical placement of each point is as follows: Zusanli is located 3 cun/ 4 fingers below the edge of the patella, specifically on the anterior muscle of the tibia. Taichong is found on the dorsum pedis, near the point where the first and second metatarsal base meets the dorsalis pedis artery. Taixi is positioned halfway between the medial malleolus and the calcaneus tendon. Lastly, Sanyinjiao is located 3 cun / 4 fingers above the medial malleolus, precisely at the posterior edge of the tibia (Fig. 2) [17].
Three professionals and twelve research assistants gave self-acupressure training. A research assistant monitors 5-6 subjects by joining the WhatsApp group. The research assistant reminded each subject to perform the exercise 3 times a week by communicating via WhatsApp. Each practice session lasted 30 minutes and each subject received a self-acupressure manual, video, and acu- pressure tools. The day after the training, the respondents performed self-acupressure exercises for 13 weeks. Monitoring and online discussions were carried out via WhatsApp media.
Finally, the DM control parameter was remeasured using the same method as before self-acupressure was performed. The DM control targets were as follows: HbA1c less than 7%, LDL cholesterol less than 100 mg/dl, BMI 18.5 – 22.9, systolic blood pressure less than 140 mmHg, diastolic blood pressure less than 90 mmHg, preprandial capillary blood glucose 80-130 mg/dl, and 2 hours postprandial (PP) capillary blood glucose less than 180 mg/dl.2
2.4. Data Analysis
To determine the effectiveness of self-acupressure, an analysis of differences in DM control parameters was carried out between before and after treatment. Because the data are numerical in nature, a difference test based on parametric statistical methods is carried out, with the requirement of normality of data distribution. The results of the data are normally distributed. The results of the normality test using the Kolmogorov-Smirnov test showed a normal distribution (p-value >0.05) found for five pairs: HbA1c, BMI, systolic pressure, diastolic pressure, and 2 hours postprandial blood glucose; therefore, the analysis of differences was performed using a paired sample t-test. Regarding fasting blood glucose and LDL, it was found that the data were not normally distributed (p-value <0.05), so the analysis of the differences was carried out using the Wilcoxon Signed Rank Test.
2.5. Confounding Variables
Age, gender, medication, diet, and activity were considered the confounding variables in this study. Indonesia has a national standard treatment of T2DM, which includes medication, diet, and activity. Every month, the public health centre would check the patient's condition. The study checked medication adherence, diet (eating time, type, and amount), and activity. The participants were obedient to medication adherence. The dynamic answers were found in the diet activity and age. We used multiple analyses of covariance (MANCOVA) to see whether there is an impact on age, diet and activity.
2.6. Ethical Considerations
The Denpasar Health Polytechnic Ethics Commission granted ethical approval with reference number LB.070/0995/IP/DPM PTSP/2023. The study permit was acquired from the Director of the Gianyar Regency Investment and One-Stop Service Office. Before gathering the pre-test data, all research participants had provided their informed consent by signing a document.
3. RESULTS
The majority (51%) of the patients were women. The patients' age ranges from 45 to 79 years, with an average age of 59.6 ± 6.28 years. The duration of the disease ranges from 6 months to 20 years, with an average of 5.8 ± 4.9 years. Table 1 provides a summary of the attainment of the objectives for each DM control measure, both before and after treatment.
| DM Control Parameters and Targets | Before Treatment | After Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Achieved | Not Achieved | Achieved | Not Achieved | |||||
| f | % | f | % | f | % | f | % | |
| HbA1C (<7%) | 13 | 52 | 12 | 48 | 13 | 52 | 12 | 48 |
| LDL (<100 mg/dl) | 3 | 12 | 22 | 88 | 11 | 44 | 14 | 56 |
| BMI (18,5-22,9 kg/m2) | 8 | 32 | 17 | 68 | 8 | 32 | 17 | 68 |
| Systolic (<140 mmHg) | 16 | 64 | 9 | 36 | 22 | 88 | 3 | 12 |
| Diastolic (<90 mmHg) | 20 | 80 | 5 | 20 | 25 | 100 | 0 | 0 |
| Fasting blood glucose (80-130 mg/dl) | 6 | 24 | 19 | 76 | 12 | 48 | 13 | 52 |
| Postprandial blood glucose (<180 mg/dl) | 5 | 20 | 20 | 80 | 6 | 24 | 19 | 76 |
*%=percentage.
| DM Control Parameters | Before Treatment | After Treatment | t or Z value | ||
|---|---|---|---|---|---|
| Mean±SD | 95% CI | Mean±SD | 95% CI | ||
| HbA1c (%) | 8.22±2.88 | 7.03-9.41 | 7.48±2.15 | 6.59-8.36 | 2.681*a |
| LDL (mg/dl) | 140±41.71 | 123.06-157.49 | 115.68±33.66 | 101.79-129.57 | 3.633**b |
| BMI (kg/m2) | 25.69±3.71 | 24.16-27.22 | 25.35±3.63 | 23.86-26.85 | 4.322**a |
| Systolic pressure (mmHg) | 132.92±14.44 | 126.96-138.88 | 124.20±11.95 | 119.27-129.13 | 4.804**a |
| Diastolic pressure (mmHg) | 77.88±12.32 | 72.79-82.97 | 76.60±1.05 | 74.23-78.96 | 0.576a |
| Fasting blood glucose (mg/dl) | 183.08±58.96 | 158.74-207.42 | 148.72±54.82 | 126.09-171.35 | 4.286**b |
| Postprandial blood glucose (mg/dl) | 230.44±70.36 | 201.39-259.49 | 211.76±50.16 | 191.05-232.47 | 1.661a |
bWilcoxon signed rank test.
*p<0.05.
**p<0.001.
The mean value of all DM control indicators evaluated in this study has shown a decrease after therapy. The average parameter values before and after therapy are compared in Table 2.
The confounding variable (age, type and amount of food) showed significant influence on ∆HbA1c, ∆LDL, and ∆postprandial blood glucose. The type of food significantly influenced ∆HbA1c (F=5.629, p=0.017) and ∆LDL (F=7.469, p=0.007). The amount of food significantly influenced ∆HbA1c (F=8.903, p=0.002) and ∆postprandial (F=4.372, p=0.025). Age significantly influenced ∆LDL (F=7.712, p=0.016). The type and amount of food were together influencing ∆HbA1c (F=7.186, p=0.019) and ∆LDL (F=6.478, p=0.024).
4. DISCUSSION
This study discovered that the mean value of all parameters decreased following the subjects' application of self-acupressure at the ST36, LR3, KI3 and SP6 points. The study's findings demonstrated that self-acupressure at the ST36, LR3, KI3, and SP6 sites, in conjunction with compliant medication, meal type, and food quantity, influenced diabetic mellitus control metrics including reduced average levels of HbA1c, LDL, BMI, systolic blood pressure, and fasting blood glucose. The paired difference test findings demonstrated significant differences in parameters HbA1c (p = 0.01), LDL (p < 0.001), BMI (p < 0.001), systolic pressure (p < 0.001), and fasting glucose levels (p < 0.001). A study conducted in Iran investigated the effects of acupressure intervention on fasting glucose levels. The study focused on specific acupressure sites, including ST-36, LR3, KI3, and SP-6. The baseline fasting glucose levels of the participants were found to be 128.30 ± 35.73. However, after acupressure treatment, these levels reduced to 122.23 ± 30.93 [15]. A study conducted in East Java investigated the effects of acupressure intervention at the ST-36 point in patients with diabetes mellitus (DM). The results showed that after 11 weeks of treatment, there was an average reduction of 240.47 ± 155.211 in random glucose levels [14]. A separate study determined that the use of acupuncture with needling at the ST-36 point was successful in lowering blood glucose levels in individuals with type 2 diabetes mellitus [18]. Multiple studies provide evidence that acupressure therapy significantly reduces blood glucose levels. Acupressure therapy enhances the induction of insulin sensitivity, as demonstrated by Huang et al. in 2016. Studies conducted on rats have shown that acupuncture at specific sites, namely, Neiguan (PC6), Zusanli (ST36) and Sanyinjiao (SP6), improves insulin sensitivity by stimulating signaling pathways, such as activation of phosphatidylinositol 3-kinase (PI3K) and GLUT-4 in skeletal muscle [19, 20]. Elevated insulin sensitivity leads to increased generation of nitric oxide (NO). Accroissement of nitric oxide (NO) synthesis has a significant effect on endothelial cell restoration and the widening of blood vessels [21]. Additional research has demonstrated that acupuncture treatment diminishes oxidative stress by decreasing nitrous oxide levels, enhancing superoxide dismutase (SOD) activity, and promoting the production of different enzymes and antioxidants. The role of oxidative stress in the development of hypertension has been demonstrated [22]. According to Ning et al., electroacupuncture performed in Zusanli (ST36) and Shenshu (BL23) resulted in an increase in GLUT 2 mRNA levels and glucokinase (GCK) activity. GLUT 2 functions as a modulator of insulin production in pancreatic beta cells in response to increased glucose levels [23, 24]. CGK exerts regulatory control over insulin secretion and glycogen synthesis in the liver, thus influencing glucose metabolism [25]. GCK functions as a “glucose sensor” that enables the pancreas to release insulin. Insulin exerts an antilipolytic effect on adipose tissue, leading to a decrease in the release of non-esterified fatty acids (NEFA). NEFA serves as a substrate for the synthesis of VLDL. Furthermore, insulin also has a crucial function in improving the production and activation of LDL receptors, thus facilitating the removal of LDL [26].
Of the seven characteristics assessed in this study, it was found that the lowest achievement in controlling DM was in the LDL control, with a target of 12%. The mean LDL level before treatment was 140.28 ± 41.72 mg/dl. The mean LDL levels of diabetic patients attending Kariadi Hospital in Semarang were reported to be 110.64 ± 32.10 mg/dL [27]. At Mangusada Badung Regional Hospital, mean LDL levels were 115.62 ± 36.36 mg/dL [28]. Another study revealed a mean LDL level of 126.20 ± 25.793 mg/dL [29]. LDL, or low-density lipoprotein, is composed of cholesterol esters (35-40%), phospholipids (20-25%), triglycerides (8-12%), free cholesterol (5-10%) and apolipoprotein B (20-24%). Within the body, there is a circulation of LDL particles that come in different sizes. The development of tiny, dense LDL particles is strongly linked to insulin resistance and hypertriglyceridemia. Cholesteryl ester transfer protein (CETP) and liver lipase help in triglyceride transfer from very low-density lipoprotein 1 (VLDL1) to LDL [30]. LDL, characterised by its tiny and compact structure, is highly susceptible to oxidation, making it an important contributor to the development of atherosclerosis. Oxidised LDL adheres easily to the blood arteries, leading to plaque formation. Plaque in blood vessels causes the lumen to constrict and reduces flexibility, leading to the disruption of blood circulation [31].
The objective of managing fasting glucose levels and glucose levels 2 hours after a meal remains insufficient, namely, 24% and 20%, respectively. A study conducted at Sanglah General Hospital Denpasar revealed that 52% of patients with diabetes mellitus had postprandial glucose levels over 180 mg/dl after 2 hours [32]. The study conducted in Surabaya found that fasting glucose levels of customers with diabetes mellitus were measured at 175.29 ± 61.38 mg / dl, while glucose levels 2 hours after a meal were measured at 208.22 ± 9.19 mg/dl [33]. The presence of glucose in the bloodstream is a result of the absorption process in the digestive system, as well as glycogenolysis and gluconeogenesis. During periods of increased absorption after a meal, a portion of the glucose will be stored in the liver and muscles. Insulin is necessary for glucose storage. Therefore, insulin secretion is controlled according to the level of glucose in the bloodstream [34]. Insulin, produced by pancreatic beta cells, attaches to insulin receptor substrates in several organs, particularly in the liver and muscles. Binding of insulin to the insulin receptor leads to an augmentation in the levels of glucose transporter (GLUT) 2 and 4. GLUT facilitates the transport of glucose from the extracellular space to the intracellular space. Postprandial hyperglycaemia occurs as a consequence of reduced insulin production and/or insulin receptor resistance. Insulin also inhibits the creation of glucose within the body. If the liver tissue exhibits insulin resistance, it leads to a decrease in the inhibitory action, increasing the processes of glycogenolysis and gluco- neogenesis. Elevated glycogenolysis and gluconeogenesis led to a rise in fasting blood glucose levels [35]. HbA1c levels have a substantial correlation with both pre-prandial and postprandial blood glucose levels [36].
The average BMI is 25.69 with a standard deviation of 3.71. A separate study discovered that the mean BMI of people with diabetes mellitus (DM) was 28.70 ± 3.46 [29] and 29.2 ± 4.1 [37]. A study conducted at the Ulee Kareng Health Centre in Banda Aceh, known as Posbindu PTM, revealed that 72.7% of people with DM were classified as overweight, according to a research conducted by Mardhatillah et al. (2022). Being overweight has a negative impact on insulin function in DM patients. Insulin resistance increases in direct proportion to body weight. Insulin resistance leads to elevated blood glucose levels (hyperglycaemia), abnormal lipid levels (dyslipidaemia), and high blood pressure (hypertension) [2, 38]. Additional research has indicated a positive correlation between body weight and the likelihood of experiencing cardiovascular events and mortality (Bangalore et al., 2018). According to Thomas, several studies have found that people with a BMI classified as obesity are 3.44 times more likely to experience lipohypertrophy [39]. BMI is a straightforward measure of the relationship between body weight and height, commonly used to categorise body weight. Currently, BMI plays a crucial role in assessing the prevalence of obesity [40]. Obesity is caused by the accumulation of fat in adipose tissue. In addition to serving as a backup energy source, adipose tissue also functions as an endocrine gland. Adipose tissue releases around 50 different types of adipokine hormones. One of the adipokines under consideration is resistin [41]. There is a direct link between resistin and BMI. Animal studies have demonstrated that resistin triggers the programmed cell death of pancreatic beta cells, a process known as apoptosis. Resistin diminishes the activation of insulin receptors and promotes the production of glucose in the liver [42]. Therefore, being overweight in DM will increase the condition.
A majority of the participants (52%) failed to achieve the desired HbA1c level, which is set below 7%. A study carried out at Wenzhou Medical University of China tertiary care hospital revealed that only 16.7% of patients with DM had HbA1c values below 7% [43]. A separate study found that 77.3% of patients with diabetes mellitus had HbA1c levels greater than 7 [9]. In case of Type 2 DM at the Jayabaru Health Centre in Banda Aceh City, the percentage was even higher at 81.2% [44]. The mean HbA1c level of the participants was 8.22 ± 2.88. A separate investigation documented that HbA1c values in patients with diabetes mellitus were found to be 8.39 ± 1.60, as reported by Chaha et al. in 2021 [29]. HbA1C is a measure that characterises the process of attaching sugar molecules to haemoglobin. HbA1c levels are influenced by the interaction between glucose concentration in the blood and the age of red blood cells. Erythrocytes have an average lifespan of 120 days. Erythrocytes undergo continuous turnover, and old cells are constantly replaced by new ones. It is expected that around 50% of HbA1c readings reflect glucose consumption in the last 30 days, while 40% reflect exposure in the previous 31-90 days, and 10% reflect exposure in the previous 91-120 days [45].
The majority of the respondents (64%) successfully achieved systolic control goals, while 80% achieved diastolic control goals. A separate study found that 39.3% of individuals with DM failed to achieve the desired blood pressure control goals [10]. A study conducted on diabetic patients receiving treatment at specialised hospitals in Northwest Ethiopia revealed that 70.3% of patients achieved systolic blood pressure levels below 140, while 87.4% of patients achieved diastolic blood pressure levels below 90 [46]. The mean systolic pressure in this study was 133.00 ± 14.44, while the mean diastolic pressure was 78.00 ± 12.32. The study conducted by Bangalore et al. found that the average systolic pressure was 138.00 ± 17.3 while the average diastolic pressure was 80.10 ± 9.30 [37]. Monitoring blood pressure is crucial for customers with diabetes mellitus. DM frequently coexists with essential hypertension. The co-occurrence of these two diseases increased susceptibility to cardiovascular system abnormalities [47]. Nevertheless, individuals with DM were discovered to have a hypertension prevalence that was twice as high as those without DM [48, 49]. Patients with DM frequently suffer from isolated systolic hypertension. This hypertension exhibits a higher level of resistance to treatment. According to the EUROASPIRE IV survey, a study conducted by Gyberg et al., only 54% of diabetic patients were able to achieve a blood pressure level below 140/90 mmHg [50]. On the other hand, hypertension can also impact the body's ability to respond to insulin. Individuals diagnosed with hypertension have a higher likelihood of acquiring DM compared to individuals with normal blood pressure [49]. However, it is important to note that insulin resistance and diabetes are not only metabolic diseases, but also increase the likelihood of developing hypertension [51]. Hypertension and diabetes mellitus are interconnected disorders.
5. LIMITATIONS
This study employed a pre-test-post-test design; however, it did not undergo comparison with other approaches. Another drawback was the autonomous execution of acupressure by patients, which may have affected its quality. Furthermore, this study did not control other factors that may have impacted the findings, for example, medication, dietary requirements, physical activity and exercise.
6. IMPLICATIONS
The study results that indicate significant improve- ments in various control parameters for diabetes mellitus due to self-acupressure have profound implications for nursing practice. Nurses can incorporate self-acupressure techniques into care plans for diabetic patients, teaching them to apply pressure to specific points, such as ST36, LR3, Taixi, and SP6 to help manage their condition. This finding supports a more holistic approach to patient care, where complementary therapies like self-acupressure are used alongside conventional treatments. Educating patients about the benefits and techniques of self-acupressure empowers them to take an active role in managing their health, improving their overall well-being and complying with their care plans.
In the realm of nursing education, the integration of complementary therapies, such as self-acupressure, into the curriculum can prepare future nurses with a wider range of skills for patient care. By incorporating modules on these therapies, nursing programmes can ensure that graduates are well equipped to provide holistic and patient-centred care. Furthermore, offering regular workshops and continuing education courses for prac- tising nurses can keep them up to date with new complementary therapies and their applications in clinical practice. Emphasising research literacy in nursing education can further enhance the ability of nurses to interpret and apply research findings, ensuring evidence-based care.
The implications for research are equally significant. There is a need for further studies to explore the mechanisms behind the effects of self-acupressure on diabetes control parameters and to validate these findings across larger and more diverse populations. Comparative studies could evaluate the effectiveness of self-acupressure against other complementary and conven- tional therapies, providing a clearer picture of its relative benefits. Longitudinal studies are also necessary to assess the long-term impact of self-acupressure on diabetes management, including potential side effects or contra- indications, ensuring a comprehensive understanding and safe practice.
Public health centres can play a crucial role in disseminating the benefits of self-acupressure. Developing programmes and workshops to teach self-acupressure techniques to diabetic patients can be an effective addition to diabetes management education. Allocating resources to create educational materials, such as brochures and videos, about self-acupressure can facilitate widespread dissemination of this knowledge in clinics and public health centres. Encouraging a collaborative care model in which public health nurses, acupuncturists, and other healthcare providers work together can ensure comprehensive diabetes management plans that include self-acupressure, promoting a holistic approach to healthcare.
In conclusion, the significant impact of self-acupressure on diabetes control parameters such as HbA1c, LDL, BMI, systolic pressure, and fasting blood glucose suggests that it can be a valuable complementary therapy.
CONCLUSION
The research findings demonstrated that the application of self-acupressure at certain locations (ST36, LR3, KI3, and SP6) in conjunction with compliant medication, dietary choices, and portion control had a significant impact on the management of diabetes mellitus. This intervention resulted in notable reductions in average levels of HbA1c, LDL cholesterol, BMI, systolic blood pressure, and fasting blood glucose. Integrating self-acupressure into nursing practice, education, and public health strategies can enhance diabetes management, improve patient outcomes, and promote a holistic approach to healthcare. By embracing these findings, healthcare professionals can offer more effective, patient-centred care and empower people to take an active role in the management of their health. The results of this research are very useful in treating patients with DM patients, so it needs to be further developed using a control group design approach.
AUTHORS' CONTRIBUTION
All authors contributed equally in terms of concept, research, implementation, analysis, and interpretation of data, as well as in the process of preparing articles. All authors shared equal responsibility for all aspects of the work in this study.
LIST OF ABBREVIATIONS
| DM | = Diabetes mellitus |
| T2DM | = Type 2 DM |
| LDL | = Low-density lipoprotein |
| HDL | = High-density lipoprotein |
| SEAM | = Study on self-acupoint massage |
| HbA1c | = Haemoglobin |
| NEFA | = Non-esterified fatty acids |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Denpasar Health Polytechnic Ethics Commission, Denpasar granted ethical approval with reference number LB.070/0995/IP/DPM PTSP/2023.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Before gathering the pre-test data, all research participants had provided their informed consent by signing a document.
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during this study are available from the corresponding author [I.S] upon request.


